Medical Device Quality Policy Examples . in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. Understanding the structure of qms. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. this guide provides a useful overview of quality management for medical devices and iso 13485 and clarifies.
from templates.rjuuc.edu.np
a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. this guide provides a useful overview of quality management for medical devices and iso 13485 and clarifies. Understanding the structure of qms. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the.
Medical Device Quality Plan Template
Medical Device Quality Policy Examples a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. Understanding the structure of qms. a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. this guide provides a useful overview of quality management for medical devices and iso 13485 and clarifies.
From soptemplates.org
Quality Policy Statement for Medical Devices Medical Device Quality Policy Examples this guide provides a useful overview of quality management for medical devices and iso 13485 and clarifies. Understanding the structure of qms. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. a quality management system (qms) for medical devices requires a plan, a manual, a policy,. Medical Device Quality Policy Examples.
From www.orthopaedic-implants.com
Quality Policy Medical Device Quality Policy Examples this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. tmc’s quality system. Medical Device Quality Policy Examples.
From www.qmswrapper.com
Quality Manual Creator and the GAP Report Tool qmsWrapper Medical Device Quality Policy Examples quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. Understanding the structure of qms. a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. this. Medical Device Quality Policy Examples.
From easymedicaldevice.com
About Us Medical Device Regulation and ISO quality standard Medical Device Quality Policy Examples in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. Understanding the. Medical Device Quality Policy Examples.
From www.pdffiller.com
Fillable Online Medical Device Quality Agreement Template Fax Email Medical Device Quality Policy Examples Understanding the structure of qms. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops.. Medical Device Quality Policy Examples.
From www.orielstat.com
Medical Device Quality Management System (QMS) Oriel STAT A MATRIX Medical Device Quality Policy Examples a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. in clause 4.2.1,. Medical Device Quality Policy Examples.
From www.examples.com
Quality Policy 18+ Examples, Google Docs, Pages, Word, PDF Medical Device Quality Policy Examples a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. this guide will walk. Medical Device Quality Policy Examples.
From www.template.net
Medical Device Quality Agreement Template in PDF, Word, Google Docs Medical Device Quality Policy Examples quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. Understanding the structure of qms. this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the. Medical Device Quality Policy Examples.
From www.specialteam.com
SpecialTeam Quality Policy Statement Medical Device Quality Policy Examples this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. Understanding the structure of qms. a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms). Medical Device Quality Policy Examples.
From iziel.com
QMS Documentation for Medical Devices ISO 13485 Certification IZiel Medical Device Quality Policy Examples this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. tmc’s quality system is specifically intended. Medical Device Quality Policy Examples.
From www.freyrsolutions.com
Quality Management System (QMS) in Medical Devices Freyr Global Medical Device Quality Policy Examples tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. Understanding the structure of qms. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu). Medical Device Quality Policy Examples.
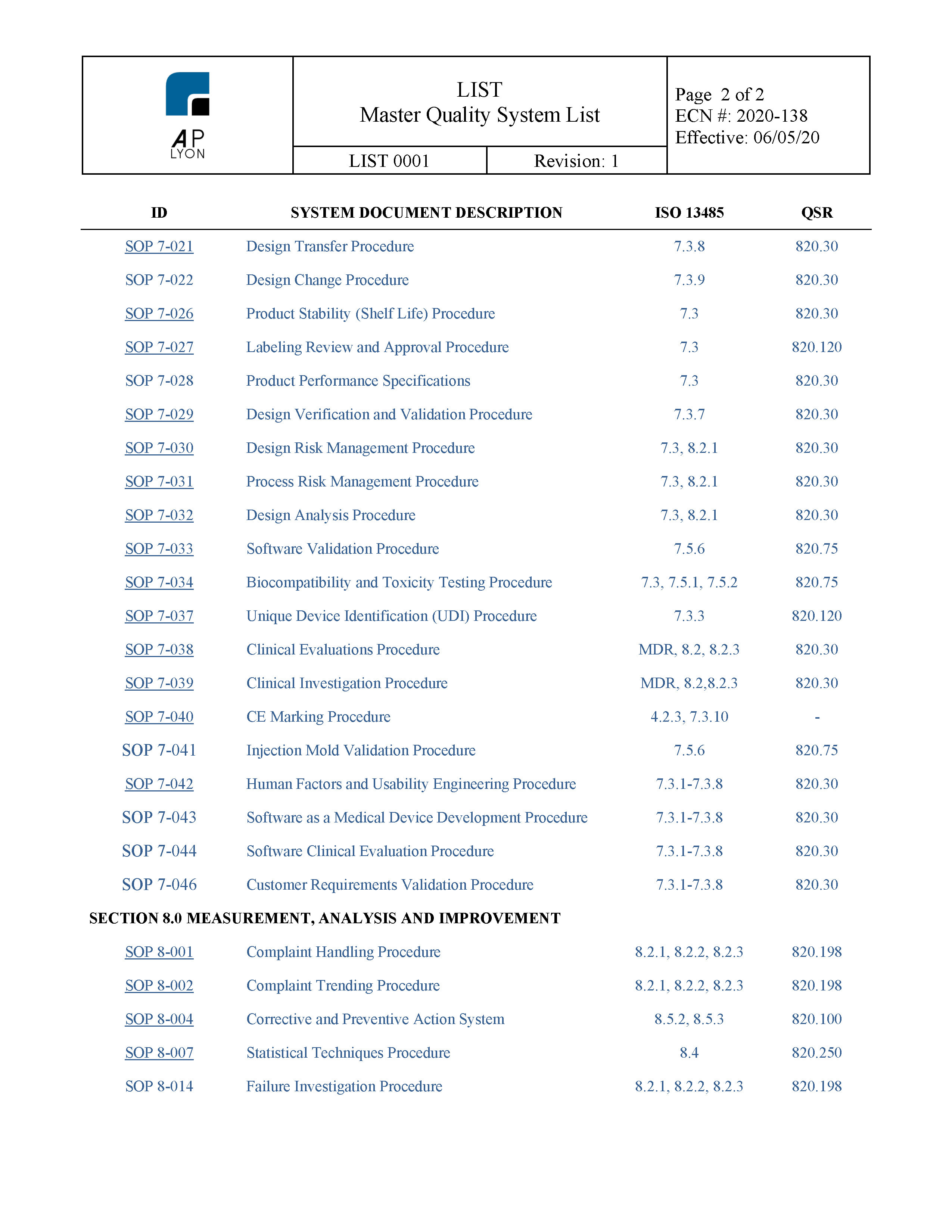
From www.aplyon.com
Medical Device Process Validation Procedure ISO 13485 and FDA QSR Medical Device Quality Policy Examples in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. . Medical Device Quality Policy Examples.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Quality Policy Examples this guide provides a useful overview of quality management for medical devices and iso 13485 and clarifies. this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. in clause 4.2.1,. Medical Device Quality Policy Examples.
From www.mitek.co.uk
Quality Policy Statement June 2020 MiTek UK and Ireland Medical Device Quality Policy Examples a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. this guide provides a useful overview of quality management for medical devices and iso 13485 and clarifies. Understanding the structure of qms. this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system.. Medical Device Quality Policy Examples.
From www.template.net
Medical Device Quality Agreement Template Google Docs, Word, Apple Medical Device Quality Policy Examples in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. this guide will. Medical Device Quality Policy Examples.
From templates.rjuuc.edu.np
Medical Device Quality Plan Template Medical Device Quality Policy Examples Understanding the structure of qms. this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system. Medical Device Quality Policy Examples.
From www.examples.com
Quality Policy 18+ Examples, Google Docs, Pages, Word, PDF Medical Device Quality Policy Examples this guide will walk you through the globally harmonized standard for medical devices iso 13485 quality management system. in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. Understanding the structure of qms. a quality management system (qms) for medical devices requires a plan, a manual, a. Medical Device Quality Policy Examples.
From medicaldevicehq.com
A guide to quality management for medical devices and ISO 13485 Medical Device Quality Policy Examples in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical. a quality management system (qms) for medical devices requires a plan, a manual, a policy, and sops. Understanding the structure of qms. quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745.. Medical Device Quality Policy Examples.